Home » Pi-Cardia received FDA market clearance for ShortCut™
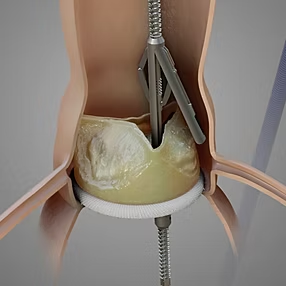
On sep 30, 2024 Pi-Cardia Ltd., a global innovator in heart valve treatment solutions, announced that the U.S. Food and Drug Administration (FDA) has granted market clearance for ShortCut™. This groundbreaking device is the first of its kind, specifically designed for leaflet modification to enable valve-in-valve Transcatheter Aortic Valve Replacement (TAVR) procedures, addressing the risk of coronary obstruction in patients. Virtual Point produced a 3D animation to showcase the MOA (Mode of Action) of the ShortCut™ device.
We Are Here For You